GIC Management System Certification Procedure
PAGE INFORMATION

CONTENT
GIC Management System Certification Procedure
GIC is a third-party certification body that has obtained accreditation for its management system certification from the U.S. accreditation body IAS; it has broad audit capabilities and certification know-how for management systems related to quality, environment, safety, health, food, and information protection, and provides management system certification services in various fields.
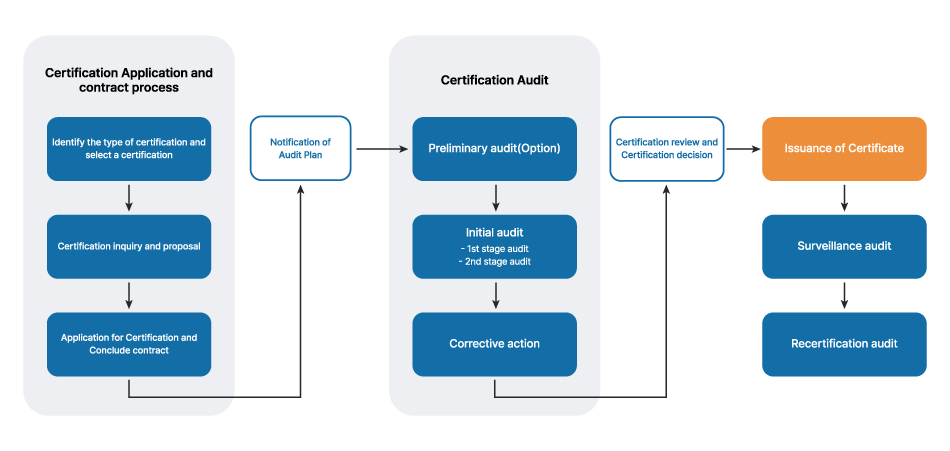 <GIC Management System Certification Procedure>
<GIC Management System Certification Procedure>
Step 1. Select a Certification
In order to acquire certification, identify the type of certification in advance, select an international standard certification that is suited for the purpose of own company.
The ISO management system certification scheme that can be conducted through GIC is as follows.
- • ISO 9001:2015 (Quality Management System)
- • ISO 14001:2015 (Environmental management systems)
- • ISO 45001:2018 (Occupational Health and Safety Management System)
- • ISO 13485:2016 (Medical devices - Quality management systems)
- • ISO/IEC 27001:2013 (Information Security Management System)
- • ISO 22000:2018 (Food Safety Management System)
- • ISO 37001:2016 (Anti-bribery management systems)
- • ISO 37301:2021 (Compliance Management System)
- • ISO 21001:2018 (Educational Organizations Management System)
- • ISO 22716:2007 (GMP - Guidelines on Good Manufacturing Practices)
** For more information, please refer to the top menu "Services → System Certification"
Step 2. Certification inquiry and proposal
Conducting consultation about the overall procedure and method about certification audit before applying for certification, and a proposal about the certification audit period and certification cost is delivered at the when the company requested.
** GIC Certification Team Contact : info@gicert.org
Step 3. Application for Certification and Conclude contract
When receipt of the application, concluded a contract automatically and when requested the contract document separately, After presenting a certification contract, the certification body must discuss certification procedures, the scope of certification, and costs with that company. At this time, certification audit mandays that determine certification costs are stipulated by international standards.
Step 4. Notification of Audit plan
After the application of certification has been confirmed, the Certification body will notify the company of the detailed audit plan included the audit team and audit schedule. At this time, discusses the audit schedule with the company.
In addition, the certification body organizes the certification audit team to be suitable for audit mandays same as specified in the contract after the certification contract is concluded. And audit that company to ensure that the management system established by the company is implemented whether suitably for international standards.
Step 5. Preliminary audit (Option)
The purpose of the Preliminary audit is to give an opportunity to the client to improve their system before the actual audit. In case of the applied company's request only, a preliminary audit will be conducted.
However, in the case of some certification bodies, a preliminary audit is sometimes conducted to evaluate whether the scope of certification applied by the company is appropriate.
Step 6. Certification audit
In the case of the initial audit, the audit will be conducted that is divided into the 1st audit (Stage 1), and the 2nd audit (On-site audit, Stage 2). In the case of the surveillance audit after it, only the 2nd audit will be conducted.
First, 1st audit (Stage 1) is conducted to confirm the suitability of the management system, and there is a partial difference in the scope of the audit by the standard applied for, and it will be reviewed about the management system document.
2nd audit (On-site audit, Stage 2) is to confirm the actual facts related to whether the certification application standards and the organization's management system meet all requirements related to the scope of certification through an on-site audit. If the requirements of the standards are met based on objective facts, certification registration can be recommended.
However, during the visit and audit on-site, if the scope of certification applied by the company is different from the facts or can't be applicable the audit team may report it to the certification body and re-conclude the certification contract by consulting the appropriate scope of certific ation with that company.
Step 7. Corrective action of non-conformity
The certification audit team tells a company and report to a certification body about the nonconformities found after the audit and the results of the comprehensive evaluation in writing (or oral). The certification body verifies the audit results of the audit team and provides the final audit results report to that company. If nonconformity is found by the company, corrective action must be completed about all nonconformities.
Step 8. Certification Review and Certification Decision
After the corrective action is completed, the certification body delivers it to the organization in charge of certification review and certification decision and decide whether to certification. Afterward, through the Certification Decision Committee, after evaluating the appropriateness of the entire process of audit, decide whether to register for certification.
Step 9. Issuance of Certificate
After the decision of certification registration is completed, the certification body provided a certificate that specified a valid period to the company that applied the certification, the initial certification audit is closed.
** GIC certificate sample and validity can be checked at the link below.
Step 10. Surveillance audit
Companies that have acquired certification must conduct at least once a year surveillance audit in accordance with the initial on-site audit procedure to verify that they continue to comply with the certification conditions.
The regular Surveillance audit cycle takes place every year from the date of the initial audit and must be conducted regularly in accordance with ISO International Regulations and IAF MD Regulations.
Step 11. Recertification audit
Companies that have been certified must undergo a recertification audit for the purpose of re-accreditation of certification every three years, the validity period of certification. The size (audit mandays) of the recertification audit is more than two-thirds of the initial certification audit and the certification procedure is similar to the initial audit.
- PREVIJCA Article 22.05.26
- NEXTGIC Certificate sample & items 21.11.11
LIST OF COMMENTS
NO COMMENTS HAVE BEEN REGISTERED.

 한국어
한국어

